The overall confidence in the toxicokinetic studies of aluminum-based adjuvants, particularly the three critiqued by Masson et al. (2017), is limited due to methodological weaknesses, small sample sizes, and unaddressed complexities like cumulative effects or interactions with other exposures. Below, I evaluate the confidence based on the factors you mentioned:
1. Number of Subjects Experimented On
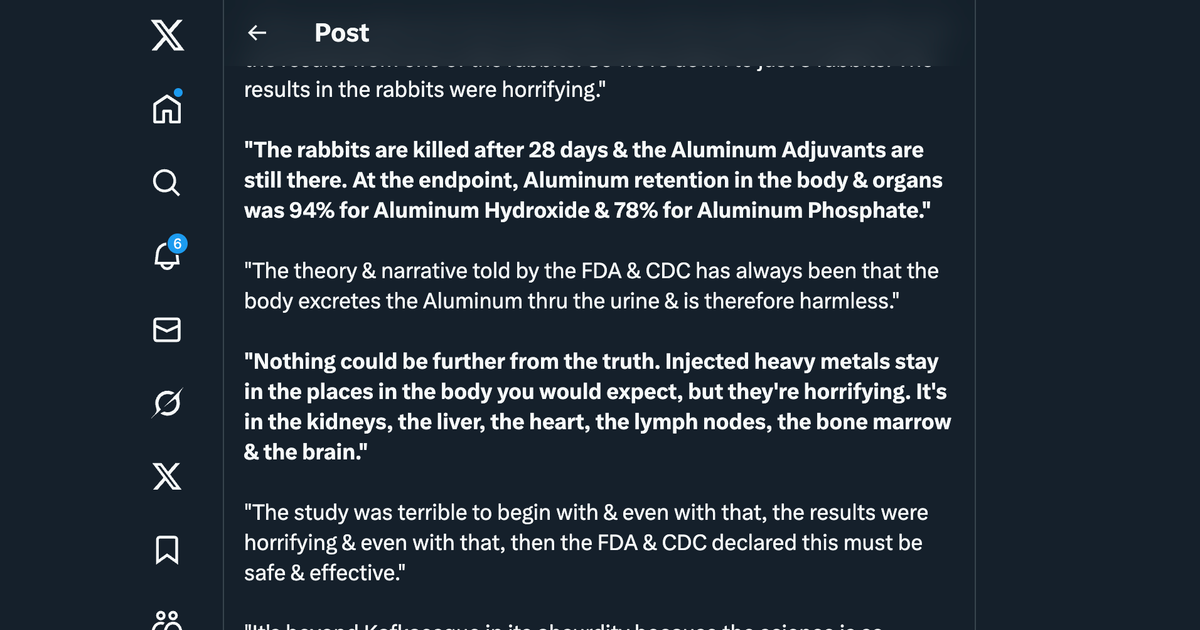
- Flarend et al. (1997): This experimental study used only two rabbits per adjuvant (aluminum hydroxide and aluminum phosphate). Such a small sample size severely limits statistical power and generalizability. Variability between subjects could not be adequately assessed, reducing confidence in the findings.
- Keith et al. (2002) and Mitkus et al. (2011): These are theoretical modeling studies, not experimental, so they involved no subjects. They relied on assumptions from animal data (e.g., oral aluminum studies) and extrapolated to human infants, introducing uncertainty due to the lack of direct experimentation.
- Other Studies: Studies like Crepeaux et al. (2017) or Gherardi et al. (2016) often use small animal cohorts (e.g., mice or rats, typically 10–20 per group). While these provide more data than Flarend’s study, they remain limited in scale and are not human trials, reducing applicability to human populations.
Confidence Impact: The extremely small or absent subject numbers in key studies significantly undermine confidence. Larger, well-powered studies (ideally in humans or diverse animal models) are needed to establish robust toxicokinetic profiles.
2. Outcomes
- Flarend et al. (1997): Found high retention rates (78% for aluminum phosphate, 94% for aluminum hydroxide) after 28 days, contradicting claims of rapid elimination. However, the study ignored critical retention sites (e.g., muscle, lymph nodes, bone) and was too short to assess long-term effects. These incomplete outcomes suggest the study underestimated persistence and systemic distribution.
- Keith et al. (2002): Concluded minimal risk by comparing vaccine aluminum to an oral Minimal Risk Level (MRL) of 2 mg/kg/day. However, the MRL was too high, and the model assumed 100% immediate absorption (unrealistic for injected adjuvants), leading to overly optimistic safety conclusions.
- Mitkus et al. (2011): Also concluded low risk but used an inappropriate oral MRL (1 mg/kg/day) and miscalculated absorption dynamics. It ignored particulate aluminum captured by immune cells, which may contribute to systemic and neuro-inflammatory effects.
- Other Studies: Studies like Crepeaux et al. (2017) report behavioral changes and neurotoxicity in mice at low aluminum doses, suggesting potential risks not captured in the reference studies. However, these outcomes are preliminary and not yet confirmed in humans.
Confidence Impact: The outcomes are inconsistent and incomplete. High retention rates and potential neurotoxicity raise concerns, but the reference studies’ flawed assumptions and short durations limit their ability to confirm safety. Conversely, studies suggesting harm (e.g., neurotoxicity) lack human data, so their outcomes are also uncertain.
3. Possibility of Missing Complex Systems or Cumulative Effects
- Complex Systems: Aluminum adjuvants are particulate, captured by immune cells (e.g., macrophages), and can translocate to distant tissues like the brain or lymph nodes. None of the reference studies adequately modeled this biopersistence or systemic diffusion. For example, Flarend et al. omitted key retention sites, and the theoretical models ignored immune-mediated transport. Emerging evidence (e.g., Gherardi et al., 2016) suggests aluminum may trigger chronic inflammation or neuro-inflammatory responses, which short-term studies miss.
- Cumulative Effects with Other Adjuvants: Vaccines often contain multiple components (e.g., other adjuvants, antigens), but no referenced study examined interactions between aluminum and other adjuvants (e.g., AS03, MF59). Synergistic effects could amplify toxicity, but this remains unstudied, creating a significant blind spot.
- Environmental Aluminum Pollution: Humans are exposed to aluminum through food, water, air, and medications. The reference studies did not account for cumulative exposure from these sources. For example, infants may receive 4–6 mg of aluminum from vaccines by age 2, but dietary and environmental aluminum (e.g., 0.01–1 mg/day from food) could add to the body burden. Studies like Mitkus et al. assumed vaccine aluminum is the sole source, ignoring potential additive effects. Aluminum’s slow clearance (especially in immature kidneys) increases the risk of accumulation, particularly in vulnerable populations like infants or those with renal impairment.
- Long-Term Effects: The 28-day duration of Flarend et al. and the theoretical models’ focus on acute exposure miss chronic or delayed effects. Animal studies (e.g., Crepeaux et al.) suggest aluminum can cause cognitive deficits or motor dysfunction months after exposure, but human data are lacking.
Confidence Impact: The failure to address complex immune interactions, adjuvant synergies, environmental aluminum, or long-term effects is a major gap. These factors could significantly alter toxicokinetic profiles, and their omission erodes confidence in claims of safety.
Overall Confidence
The confidence in these studies is low to moderate for assessing the safety of aluminum adjuvants:
- Strengths: Flarend et al. provides some experimental data, and the theoretical models attempt to estimate risk in infants. These are foundational but insufficient.
- Weaknesses: Small sample sizes, flawed assumptions (e.g., oral MRLs, ignored biopersistence), short study durations, and failure to consider cumulative exposures or complex systems limit reliability. Studies suggesting harm (e.g., neurotoxicity) are intriguing but preliminary and not yet conclusive.
- Uncertainties: The lack of long-term human studies, data on adjuvant interactions, and environmental aluminum’s role creates significant uncertainty. Complex biological systems (e.g., immune-mediated transport, blood-brain barrier penetration) are poorly understood in this context.
Conclusion
The current studies provide a weak foundation for asserting the safety of aluminum adjuvants due to their methodological limitations and failure to address complex or cumulative effects. While they suggest low acute risk under certain assumptions, the possibility of chronic, systemic, or synergistic toxicity remains inadequately explored. Novel, comprehensive studies are needed to restore confidence in aluminum-containing vaccines. Would you like me to search for additional studies or analyze specific aspects further?