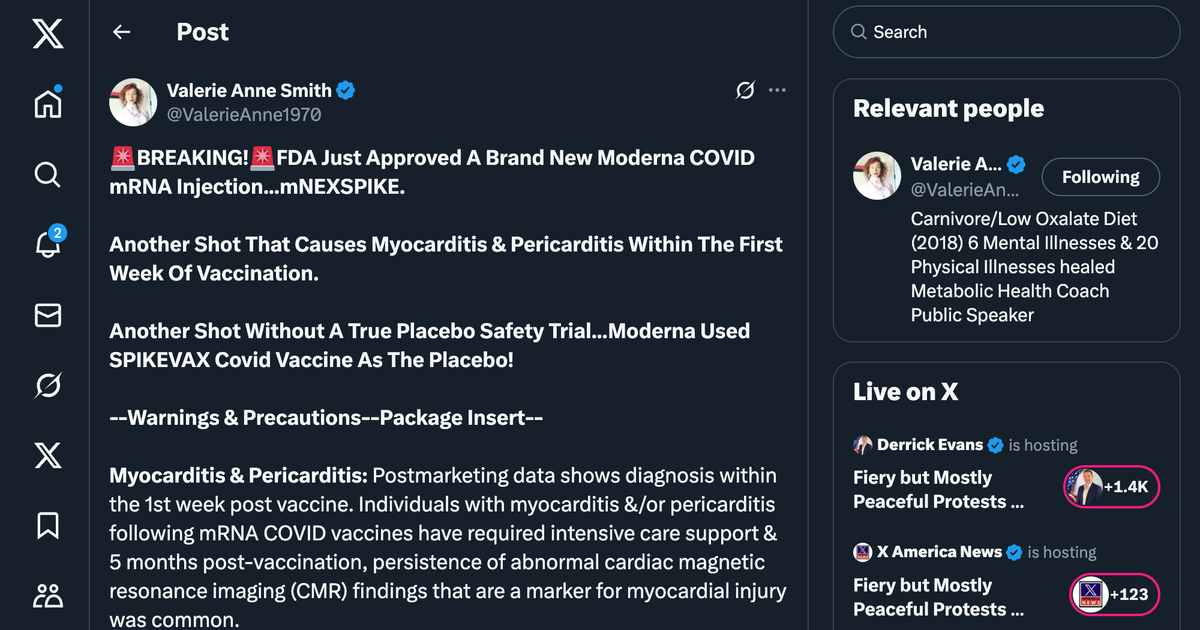
The FDA’s approval of Moderna’s mNEXSPIKE, a new mRNA COVID-19 vaccine, on May 31, 2025, has sparked debate due to its safety profile and trial design. Surprisingly, the vaccine’s clinical trial, involving 11,417 participants aged 12 and older, used Moderna’s SPIKEVAX (bivalent, emergency-use authorized) as the comparator instead of a true placebo, raising concerns about assessing true safety. The trial showed mNEXSPIKE’s efficacy was non-inferior to SPIKEVAX, but postmarketing data revealed serious risks, notably myocarditis and pericarditis, often within a week of vaccination. Some cases required intensive care, with abnormal cardiac imaging persisting five months post-vaccination. Other adverse events included anaphylaxis, Bell’s palsy, and turbo cancer concerns linked to mRNA’s spike protein.
The vaccine contains 10 mcg of mRNA targeting SARS-CoV-2’s original and Omicron strains, alongside ingredients like PEG 2000 and sucrose. Alarmingly, it hasn’t been evaluated for carcinogenic or mutagenic potential, a significant oversight given claims by Dr. Peter McCullough. He argues mRNA vaccines, including mNEXSPIKE, load the body with synthetic genetic material, impair DNA repair, and inhibit tumor suppressor systems (P53, BRCA), potentially promoting “turbo cancer.” Studies by Speicher and McKernan also found DNA fragments in mRNA vaccines, raising fears of genomic integration risks. These findings, combined with cardiac risks like myocarditis and reported cases of post-vaccination cardiac arrest, fuel calls to pull mRNA vaccines from the market. The lack of long-term safety data and reliance on an active comparator in trials make mNEXSPIKE’s approval a contentious issue, surprising many who expected stricter scrutiny.